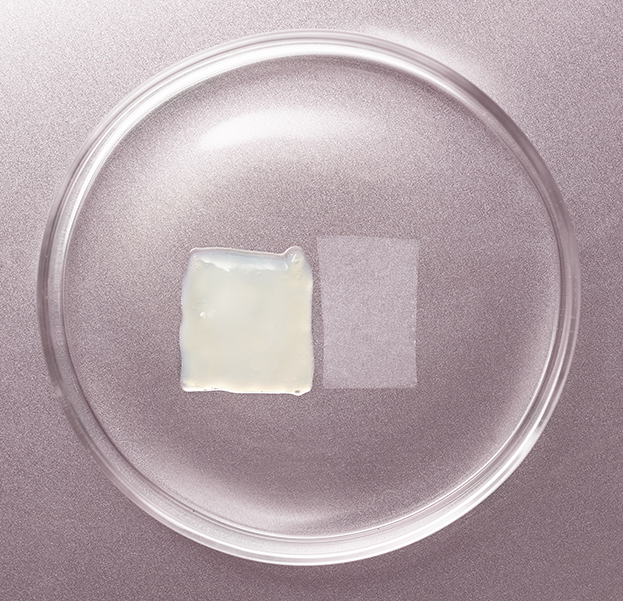
Surgical
Clarix 1K
The Power of a Thicker Graft.
Clarix® 1K is a cryopreserved, ultra-thick human amniotic membrane allograft used as an adjunct for surgical applications.
Technology
Preserving as Much of Mother Nature as Possible
The BioTissue CryoTek® cryopreservation process is the only tissue processing method shown to produce a matrix comparable to the native tissue.1,2 In conventional heat dehydration processing, critical biological components—including the majority of HC-HA/PTX3 naturally found in birth tissue—are degraded, which may limit the tissue’s healing capabilities.
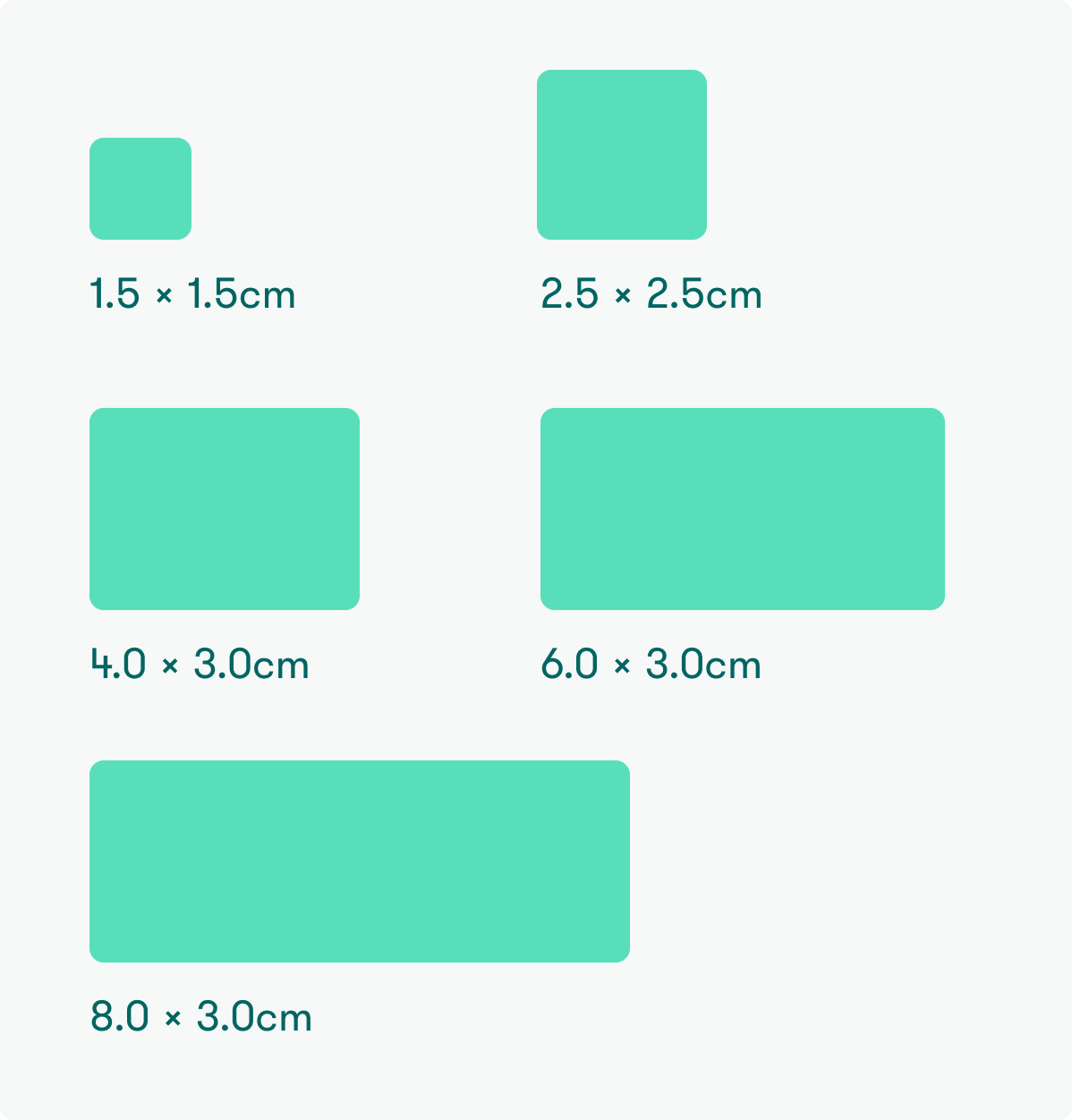
Available sizes
CR-10-1515 (1.5 x 1.5 cm)
CR-10-2525 (2.5 x 2.5 cm)
CR-10-4030 (4.0 x 3.0 cm)
CR-10-6030 (6.0 x 3.0 cm)
CR-10-8030 (8.0 x 3.0 cm)
For a wide array of surgical applications including:
- Complex Bone and Joint Reconstruction
- Soft Tissue Repair and Reconstruction
- Nerve Repair and Decompression
- Joint Arthroplasty and Arthrodesis
- Cartilage Repair
- Fractures and Non-unions
- Traumatic Wounds and Reconstruction
- Surgical Wound Healing and Dehiscence
Location & Temperature
-80°C → 4°C (-112°F → 39.2°F)
Example: ultra-low temperature freezer, standard freezer, or standard refrigerator
Shelf Life
Use within the expiration date printed on product packaging (2 years from date of manufacture).
Clarix 1K exposed to controlled room temperature (20°C to 25°C, 68°F to 77°F) for up to 6 hours may be returned to cold temperature storage in accordance with the Storage section as long as the packaging remains unopened and intact.
For Clarix reimbursement information, visit the Reimbursement page.
For case studies, register for the Physician Portal.
BioTissue Surgical Customer Support:
Call us Monday – Friday: 9:00 a.m. to 7:00 p.m. Eastern Standard Time.
Email: [email protected]
Call: (888) 709-2140
Fax: (770) 874-5563
For more information, visit our Ordering page.
To learn more about Clarix, fill out the details below:
Download Clarix 1K Product Information
Clarix 1K
Mother Nature’s natural gift of healing so patients can get back to the life they love.